Blood-brain barrier (BBB) is the mechanism that controls the passage of substances from the blood into the cerebrospinal fluid and, thus, into the brain and spinal cord. The BBB lets essential metabolites, such as Oxygen and glucose, pass from the blood to the brain and central nervous system (CNS) but blocks most molecules that are more massive than about 500 Daltons. This is a low mass in biomolecular terms and means that everything from hormones and neurotransmitters to viruses and bacteria are refused access to the brain by the BBB. It also means that many drugs, which would otherwise be capable of treating CNS disorders, are denied access to the very regions where they would be affective.
- Key functions of the BBB are:
- Protecting the brain from "foreign substances" in the blood that could injure the brain.
- Shielding the brain from hormones and neurotransmitters in the rest of the body.
- Maintaining a constant environment (homoestasis) for the brain.
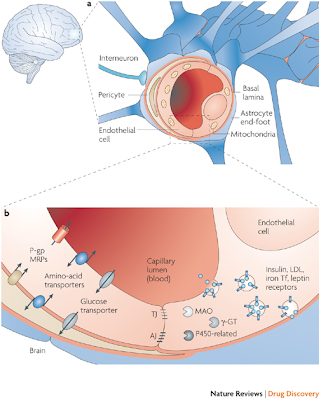
a | The blood–brain barrier (BBB) is formed by endothelial cells at the level of the cerebral capillaries. These endothelial cells interact with perivascular elements such as basal lamina and closely associated astrocytic end-feet processes, perivascular neurons (represented by an interneuron here) and pericytes to form a functional BBB. b | Cerebral endothelial cells are unique in that they form complex tight junctions (TJ) produced by the interaction of several transmembrane proteins that effectively seal the paracellular pathway. These complex molecular junctions make the brain practically inaccessible for polar molecules, unless they are transferred by transport pathways of the BBB that regulate the microenvironment of the brain. There are also adherens junctions (AJ), which stabilize cell–cell interactions in the junctional zone. In addition, the presence of intracellular and extracellular enzymes such as monoamine oxidase (MAO), γ-glutamyl transpeptidase (γ-GT), alkaline phosphatase, peptidases, nucleotidases and several cytochrome P450 enzymes endow this dynamic interface with metabolic activity. Large molecules such as antibodies, lipoproteins, proteins and peptides can also be transferred to the central compartment by receptor-mediated transcytosis or non-specific adsorptive-mediated transcytosis. The receptors for insulin, low-density lipoprotein (LDL), iron transferrin (Tf) and leptin are all involved in transcytosis. P-gp, P-glycoprotein; MRP, multidrug resistance-associated protein family.
Discovery of the BBB:
The special properties of the BBB were first observed in the late 19th century by the German bacteriologist Paul Ehrlich. He found that when he injected coloured dyes into the blood stream they leaked out of capillaries in most regions of the body to stain the surrounding tissues; the brain, however, remained unstained. Ehrlich wrongly surmised that the brain had a low affinity for the dyes. It was his student, Edwin Goldmann, who did the other half of the experiment and realized the truth of what was going on. Goldmann injected a dye into the cerebrospinal fluid that surrounds the brain and observed that it stained the brain, but nothing else. Goldmann correctly concluded that the dyes was unable to cross the specialized walls of brain capillaries.
Anatomy of the BBB:
The key aspect of the BBB is the presence of the thin, flat cells known as Endothelial cells which form the walls of capillaries. In most the body, the Endothelial cells in the capillaries overlap at what is called junctions. These junctions are leaky enough to let a lot of different materials move through the wall of the blood vessel into the tissue and back again. These materials include normally beneficial stuff such as hormones and nutrient molecules as well as potentially harmful agents like toxins, viruses, and bacteria. Substances can get into the surrounding tissues either by leaking out of the junctions or passing straight through the Endothelial cells.
However, in the brain there is a different arrangement where the Endothelial cells joins up. The Endothelial cells meet each other at what is called TIGHT JUNCTIONS. These junctions block the passage of most things except for small molecules and are a crucial components of the BBB.
In order to traverse the walls of the brain capillaries, substances must move through the Endothelial cell membranes. Because the main constitute of cell membranes are lipids, it would seem that a molecule could only can get into the brain if it was lipid-soluble. However, many ions and small molecules that are not readily soluble is lipids do move quite readily from brain capillaries into brain tissue. A molecule like glucose, the primary source of metabolic energy for neurons and glial cells, is an obvious example. The explanation for this is the presence of specific transporters for glucose and other critical molecules and ions.
In addition to tight junctions, the "end feet" of Astrocytes (Astraglia)[ the terminal regions of astrocytic processes] surround the outside of capillary Endothelial cells.
Barriers are present at three main sites: the brain endothelium forming the blood–brain barrier (BBB) (1), the arachnoid epithelium (2) forming the middle layer of the meninges, and the choroid plexus epithelium (3), which secretes cerebrospinal fluid (CSF). At each site, the physical barrier is caused by tight junctions that reduce the permeability of the paracellular (intercellular cleft) pathway. In circumventricular organs (CVOs, not shown), which contain neurons specialized for neurosecretion and/or chemosensitivity, the endothelium is leaky. This allows tissue–blood exchange, but as these sites are separated from the rest of the brain by an external glial barrier, and from CSF by a barrier at the ependyma, CVOs do not form a leak across the BBB. Modified, with permission, from Ref.163 © (1990) Kluwer Academic.
Cellular constituents of the blood–brain barrier
The barrier is formed by capillary endothelial cells, surrounded by basal lamina and astrocytic perivascular endfeet. Astrocytes provide the cellular link to the neurons. The figure also shows pericytes and microglial cells. a | Brain endothelial cell features observed in cell culture. The cells express a number of transporters and receptors, some of which are shown. EAAT1–3, excitatory amino acid transporters 1–3; GLUT1, glucose transporter 1; LAT1, L-system for large neutral amino acids; Pgp, P-glycoprotein. b | Examples of bidirectional astroglial–endothelial induction necessary to establish and maintain the BBB. Some endothelial cell characteristics (receptors and transporters) are shown. 5-HT, 5-hydroxytryptamine (serotonin); ANG1, angiopoetin 1; bFGF, basic fibroblast growth factor; ET1, endothelin 1; GDNF, glial cell line-derived neurotrophic factor; LIF, leukaemia inhibitory factor; P2Y2, purinergic receptor; TGF , transforming growth factor-
, transforming growth factor- ; TIE2, endothelium-specific receptor tyrosine kinase 2. Data obtained from astroglial–endothelial co-cultures and the use of conditioned medium.
; TIE2, endothelium-specific receptor tyrosine kinase 2. Data obtained from astroglial–endothelial co-cultures and the use of conditioned medium.
Molecular composition of endothelial tight junctions
Simplified and incomplete scheme showing the molecular composition of endothelial tight junctions. Occludin and the claudins — proteins with four transmembrane domains and two extracellular loops — are the most important membranous components. The junctional adhesion molecules (JAMs) and the endothelial selective adhesion molecule (ESAM) are members of the immunoglobulin superfamily. Within the cytoplasm are many first-order adaptor proteins, including zonula occludens 1, 2 and 3 (ZO-1–3) and Ca2+-dependent serine protein kinase (CASK), that bind to the intramembrane proteins. Among the second-order adaptor molecules, cingulin is important, and junction-associated coiled-coil protein (JACOP) may also be present. Signalling and regulatory proteins include multi-PDZ-protein 1 (MUPP1), the partitioning defective proteins 3 and 6 (PAR3/6), MAGI-1–3 (membrane-associated guanylate kinase with inverted orientation of protein–protein interaction domains), ZO-1-associated nucleic acid-binding protein (ZONAB), afadin (AF6), and regulator of G-protein signalling 5 (RGS5). All of these adaptor and regulatory/signalling proteins control the interaction of the membranous components with the actin/vinculin-based cytoskeleton. In epithelial cells, tight and adherens junctions are strictly separated from each other, but in endothelial cells these junctions are intermingled. The most important molecule of endothelial adherens junctions is vascular endothelial cadherin (VE-cadherin). In addition, the platelet–endothelial cell adhesion molecule (PECAM) mediates homophilic adhesion. The chief linker molecules between adherens junctions and the cytoskeleton are the catenins, with desmoplakin and p120 catenin (p120ctn) also involved. Itch, E3 ubiquitin protein ligase. Modified, with permission, from Ref. 20 © (2005) Wiley-VCH.
General Properties of the BBB
- Large molecules do not pass through the BBB easily.
- Low lipid (fat) soluble molecules do not penetrate into the brain. However, lipid soluble molecules, such as barbituate drugs, rapidly cross through into the brain.
- Molecules that have a high electrical charge are slowed.
What can weaken BBB ?
- Hypertension (high blood pressure): high blood pressure opens the BBB.
- Development: the BBB is not fully formed at birth.
- Hyperosmolitity: a high concentration of a substance in the blood can open the BBB.
- Microwaves: exposure to microwaves can open the BBB.
- Radiation: exposure to radiation can open the BBB.
- Infection: exposure to infectious agents can open the BBB.
- Trauma, Ischemia, Inflammation, Pressure: injury to the brain can open the BBB.
There are several areas of the brain where the BBB is weak. This allows substances to cross into the brain somewhat freely. These areas are known as "circumventricular organs". Through the circumventricular organs the brain is able to monitor the makeup of the blood. The circumventricular organs include:
* Pineal body: Secretes melatonin and neuroactive peptides. Associated with circadian rhythms.
* Neurohypophysis (posterior pituitary): Releases neurohormones like oxytocin and vasopressin into the blood.
* Area postrema: "Vomiting center": when a toxic substance enters the bloodstream it will get to the area postrema and may cause the animal to throw up. In this way, the animal protects itself by eliminating the toxic substance from its stomach before more harm can be done.
* Subfornical organ: Important for the regulation of body fluids.
* Vascular organ of the lamina terminalis: A chemosensory area that detects peptides and other molecules.
* Median eminence: Regulates anterior pituitary through release of neurohormones.
Pathophysiology:
The blood–brain barrier acts very effectively to protect the brain from many common bacterial infections. Thus, infections of the brain are very rare. However, since antibodies and antibiotics are too large to cross the blood–brain barrier, infections of the brain that do occur are often very serious and difficult to treat. However, the blood–brain barrier becomes more permeable during inflammation. This allows some antibiotics and phagocytes to move across the BBB; although, this can allow bacteria/viruses to also move across.
Astroglial–endothelial signalling under pathological conditions
Examples of astroglial–endothelial signalling in infection or inflammation, stroke or trauma, leading to opening of the blood–brain barrier (BBB) and disturbance of brain function. bradykinin, produced during inflammation in stroke or brain trauma, acts on endothelial and astroglial bradykinin B2 receptors, leading to an increase in the concentration of intracellular Ca2+. In astrocytes, this can trigger the production of interleukin-6 (IL-6) through activation of nuclear factor- B (NF-
B (NF- B) (1). Bradykinin, substance P, 5-hydroxytryptamine (5-HT, serotonin) and histamine acting on astrocytes can lead to the formation of ATP and prostaglandins (PGs), with effects on vascular tone and endothelial permeability (2) by mechanisms that are known to involve endothelium. Lipopolysaccharide (LPS), formed in infections, leads to the release from microglia of tumour necrosis factor-
B) (1). Bradykinin, substance P, 5-hydroxytryptamine (5-HT, serotonin) and histamine acting on astrocytes can lead to the formation of ATP and prostaglandins (PGs), with effects on vascular tone and endothelial permeability (2) by mechanisms that are known to involve endothelium. Lipopolysaccharide (LPS), formed in infections, leads to the release from microglia of tumour necrosis factor- (TNF
(TNF ), IL-1
), IL-1 and reactive oxygen species (including O2
and reactive oxygen species (including O2 -), all of which have the ability to open the BBB (3). Astrocytes downregulate tissue plasminogen activator (tPA) production via transforming growth factor-
-), all of which have the ability to open the BBB (3). Astrocytes downregulate tissue plasminogen activator (tPA) production via transforming growth factor- (TGF
(TGF ), but there is still sufficient tPA to open the BBB, leading to an influx of tPA from the blood (4). Following disruption of the BBB involving a decrease in agrin expression, K+ and glutamate (Glu) from the blood can reach the brain extracellular space. Aquaporin 4 (AQP4) is upregulated on the astroglial endfeet, leading to astroglial swelling (5). ET1, endothelin 1.
), but there is still sufficient tPA to open the BBB, leading to an influx of tPA from the blood (4). Following disruption of the BBB involving a decrease in agrin expression, K+ and glutamate (Glu) from the blood can reach the brain extracellular space. Aquaporin 4 (AQP4) is upregulated on the astroglial endfeet, leading to astroglial swelling (5). ET1, endothelin 1.
Meningitis
Meningitis is an inflammation of the membranes that surround the brain and spinal cord. Meningitis is most commonly caused by infections with various pathogens, examples of which are Streptococcus pneumoniae and Haemophilus influenza. When the meninges are inflamed, the blood–brain barrier may be disrupted. This disruption may increase the penetration of various substances (including either toxins or antibiotics) into the brain. Antibiotics used to treat meningitis may aggravate the inflammatory response of the central nervous system by releasing neurotoxins from the cell walls of bacteria-like lipopolysaccharide (LPS). Depending on the causative pathogen, whether it is bacterial, fungal, or protozoan, treatment with 3rd generation or 4th generation cephalosporins or amphotericin B is usually prescribed.
De Vivo disease
De Vivo disease (also known as GLUT1 deficiency syndrome) is a rare condition caused by inadequate transportation of the sugar, glucose, across the blood–brain barrier, resulting in developmental delays and other neurological problems. Genetic defects in glucose transporter type 1 (GLUT1) appears to be the primary cause of De Vivo disease.
Pathways across the BBB:
A schematic diagram of the endothelial cells that form the blood–brain barrier (BBB) and their associations with the perivascular endfeet of astrocytes. The main routes for molecular traffic across the BBB are shown. a | Normally, the tight junctions severely restrict penetration of water-soluble compounds, including polar drugs. b | However, the large surface area of the lipid membranes of the endothelium offers an effective diffusive route for lipid-soluble agents. c | The endothelium contains transport proteins (carriers) for glucose, amino acids, purine bases, nucleosides, choline and other substances. Some transporters are energy-dependent (for example, P-glycoprotein) and act as efflux transporters. AZT, azidothymidine. d | Certain proteins, such as insulin and transferrin, are taken up by specific receptor-mediated endocytosis and transcytosis. e | Native plasma proteins such as albumin are poorly transported, but cationization can increase their uptake by adsorptive-mediated endocytosis and transcytosis. Drug delivery across the brain endothelium depends on making use of pathwaysb–e; most CNS drugs enter via route b. Modified, with permission, from Ref. 8 © (1996) Elsevier Science.
Ref:
- http://www.nature.com/nrn/journal/v7/n1/fig_tab/nrn1824_ft.html
- http://faculty.washington.edu/chudler/bbb.html
- http://www.daviddarling.info/encyclopedia/B/blood-brain_barrier.html
- http://www.nature.com/nrd/journal/v6/n8/full/nrd2368.html
- http://en.wikipedia.org/wiki/Blood%E2%80%93brain_barrier
- http://newspaper.li/alpha-lipoic-acid/
- http://healthheaven.myblog.sg/2008/07/05/antioxidants-that-cross-the-blood-brain-barrier/
- http://www.eje-online.org/content/150/5/627.full.pdf+html
- http://www.springerlink.com/content/85wvbf1l8xnpu8ff/?MUD=MP








No comments:
Post a Comment