G protein-coupled receptors, often abbreviated GPCRs, are an abundant superfamily of proteins also known as seven-transmembrane domain receptors, 7TM receptors, 7 pass transmembrane receptors, heptahelical receptors, serpentine receptor, and G protein-linked receptors (GPLRs). G protein-coupled receptors are cell surface signalling proteins involved in many physiological functions and in multiple diseases.
The presence of GPCRs in the genomes of bacteria, yeast, plants, nematodes and other invertebrate groups argues in favor of a relatively early evolutionary origin of this group of molecules. The diversity of GPCRs is dictated both by the multiplicity of stimuli to which they respond, as well as by the variety of intracellular signalling pathways they activate. These include light, neurotransmitters, odorants, biogenic amines, lipids, proteins, amino acids, hormones, nucleotides, chemokines and, undoubtedly, many others. In addition, there are at least 18 different human G-alpha proteins to which GPCRs can be coupled (Hermans, 2003; Wong, 2003). These G-alpha proteins form heterotrimeric complexes with G-beta subunits, of which there are at least 5 types, and G-gamma subunits, of which there are at least 11 types (Hermans, 2003).
How do GPCRs work?
1*The first step in signal transduction is ligand (shown in Golden color in the above fig.) binding.
2*Agonist binding is followed by a change in the conformation of the receptor that may involve disruption of a strong ionic interaction between the third and sixth transmembrane helices (Ballesteros et al., 2001; Shapiro et al., 2002), which facilitates activation of the G-protein heterotrimer.
3*Depending on the type of G protein to which the receptor is coupled, a variety of downstream
signalling pathways can be activated (reviewed by Marinissen and Gutkind, 2001; Neves et al., 2002).
4*Signalling is then attenuated as following:
GPCRs become desensitized when exposed to their ligand for a prolonged period of time. There are two recognized forms of desensitization:
1) Homologous desensitization, in which the activated GPCR is downregulated
2) Heterologous desensitizationc (cross-desensitisation), wherein the activated GPCR causes downregulation of a different GPCR. The key reaction of this downregulation is the phosphorylation of the intracellular receptor domain by protein kinases.
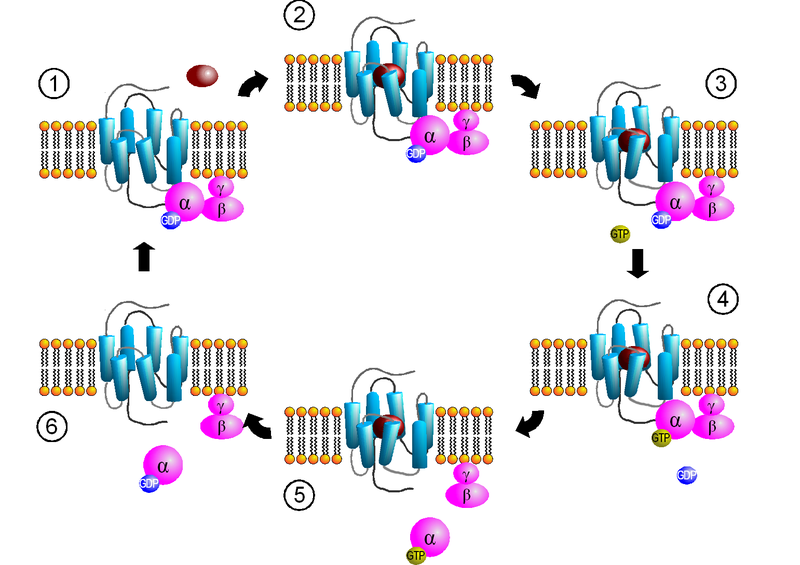
Ligand + GPCR = conformation change to GPCR = α subunit-GTP + beta-gamma complex = α subunit-GTP activates a specific effector (such as Adenylyl cyclase, PLC, ion channel, phosphodiesteras) = production of second messengers (such as cAMP, DAG, sodium and calcium conc. changes) = physiological response by controlling cellular functions = α subunit-GTP + Regulatory of G protein signalling (RGSs) =[ α subunit-GDP + beta-gamma complex] (GPCR)

-Phosphorylation by cAMP-dependent protein kinases
Cyclic AMP-dependent protein kinases (protein Kinase A) are activated by the signal chain coming from the G protein (that was activated by the receptor) via Adenylyl cyclase and cAMP. In a feedback mechanism, these activated kinases phosphorylate the receptor. The longer the receptor remains active, the more kinases are activated, the more receptors are phosphorylated.
-Phosphorylation by GRKs
GRKs-mediated receptor phosphorylation rapidly initiates profound impairment of receptor signaling and desensitization. Activity of GRKs and subcellular targeting is tightly regulated by interaction with receptor domains, G protein subunits, lipids, anchoring proteins and calcium-sensitive proteins.
G protein:
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins involved in transmitting chemical signals originating from outside a cell into the inside of the cell. G proteins function as molecular switches. Their activity is regulated by factors that control their ability to bind to and hydrolyze Guanosine triphosphate (GTP) to Guanosine diphosphate (GDP). When they bind GTP, they are 'on', and, when they bind GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases.


GDP GTP
There are two classes of G proteins. The first function as monomeric small GTPases while the second form and function as heterotrimeric G protein complexes. The latter class of complexes are made up of alpha (α), beta (β) and gamma (γ) subunits. In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex.
Note: Small GTPases are a family of hydrolase enzymes that can bind and hydrolyze GTP. They are a form of G proteins found in the cytosol which are homologous to the alpha subunit of heterotrimeric G protein complexes, but unlike the alpha subunit of G proteins, a small GTPase can function independently as a hydrolase enzyme to bind to and hydrolyze a GTP to form GDP. The most well-known members are the Ras GTPases and hence they are sometimes called Ras superfamily GTPases.
G proteins are molecular switches that use GDP (colored purple here) to control their signaling cycle. When GDP is bound, as shown here, the G protein is inactive. To activate the protein, the GDP is replaced with GTP, the G protein will deliver its signal. G proteins come in many shapes and sizes. Most are used for cell signaling, but other types play an important role in other tasks, such as powering protein synthesis. The ones described here are termed heterotrimeric G proteins because they are composed of three different chains, denoted as alpha (tan), beta (blue), and gamma (green). The little red piece is a loop on the surface of the alpha subunit that is important in transmitting the signal.

G protein:
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins involved in transmitting chemical signals originating from outside a cell into the inside of the cell. G proteins function as molecular switches. Their activity is regulated by factors that control their ability to bind to and hydrolyze Guanosine triphosphate (GTP) to Guanosine diphosphate (GDP). When they bind GTP, they are 'on', and, when they bind GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases.


GDP GTP
There are two classes of G proteins. The first function as monomeric small GTPases while the second form and function as heterotrimeric G protein complexes. The latter class of complexes are made up of alpha (α), beta (β) and gamma (γ) subunits. In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex.
Note: Small GTPases are a family of hydrolase enzymes that can bind and hydrolyze GTP. They are a form of G proteins found in the cytosol which are homologous to the alpha subunit of heterotrimeric G protein complexes, but unlike the alpha subunit of G proteins, a small GTPase can function independently as a hydrolase enzyme to bind to and hydrolyze a GTP to form GDP. The most well-known members are the Ras GTPases and hence they are sometimes called Ras superfamily GTPases.
G proteins are molecular switches that use GDP (colored purple here) to control their signaling cycle. When GDP is bound, as shown here, the G protein is inactive. To activate the protein, the GDP is replaced with GTP, the G protein will deliver its signal. G proteins come in many shapes and sizes. Most are used for cell signaling, but other types play an important role in other tasks, such as powering protein synthesis. The ones described here are termed heterotrimeric G proteins because they are composed of three different chains, denoted as alpha (tan), beta (blue), and gamma (green). The little red piece is a loop on the surface of the alpha subunit that is important in transmitting the signal.

Some Types of Gα Subunits:
#The family consists of the G protein α subunit, which acts as a weak GTPase. G protein classes are defined based on the sequence and the function of their α subunits:
Gαs:
"S" stands for stimulation since it stimulates cAMP-dependent pathway via activating Adenylyl cyclase. It is associated with the receptors for many hormones such as (Adrenaline, Glucagon, LH, PTH, ACTH).
Note: Gαs is the target of the toxin liberated by Vibrio cholerae. Therefore, Binding of cholera toxin to Gαs keeps it turned "on". The resulting continuous high levels of cAMP causes a massive loss of salts from the cells of the intestinal epithelium. Massive amounts of water follow by osmosis causing a diarrhea that can be fatal if the salts and water are not quickly replaced.
The cAMP Dependent Pathway is used as a signal transduction pathway for many hormones including:
- ADH - Promotes water retention by the kidneys (V2 Cells of Posterior Pituitary)
- GHRH - Stimulates the synthesis and release of GH (Somatotroph Cells of Anterior Pituitary)
- GHIH - Inhibits the synthesis and release of GH (Somatotroph Cells of Anterior Pituitary)
- CRH - Stimulates the synthesis and release of ACTH (Anterior Pituitary)
- ACTH - Stimulates the synthesis and release of Cortisol (zona fasiculata of adrenal cortex in kidneys)
- TSH - Stimulates the synthesis and release of a majority of T4 (Thyroid Gland)
- LH - Stimulates follicular maturation and ovulation in women; Stimulates testosterone production and spermatogenesis in men
- FSH - Stimulates follicular development in women; Stimulates spermatogenesis in men
- PTH - Increases blood calcium levels (PTH1 Receptor: Kidneys and Bone; PTH2 Receptor: Central Nervous system, Bones, Kidneys, Brain)
- Calcitonin - Decreases blood calcium levels (Calcitonin Receptor: Intestines, Bones, Kidneys, Brain)
- Glucagon - Stimulates glycogen breakdown (liver)
- hCG - Promotes cellular differentiation; Potentially involved in apoptosis
Ref:
http://inbehindthedarkness.blogspot.com/p/ce.html
http://inbehindthedarkness.blogspot.com/p/ce.html
1. http://proteopedia.org/wiki/index.php/G_protein-coupled_receptor
2. http://jcs.biologists.org/content/116/24/4867.full.pdf+html
3. http://en.wikipedia.org/wiki/G_protein-coupled_receptor
4. http://www.wjgnet.com/1007-9327/12/7753.pdf
5. http://www.rcsb.org/pdb/101/motm.do?momID=58
6. http://en.wikipedia.org/wiki/G_protein
7. http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/C/CellSignaling.html#Introduction
4. http://www.wjgnet.com/1007-9327/12/7753.pdf
5. http://www.rcsb.org/pdb/101/motm.do?momID=58
6. http://en.wikipedia.org/wiki/G_protein
7. http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/C/CellSignaling.html#Introduction



